Cell-free Synthetic Biology takes use of biomolecular reactions outside cells to synthesise specific products with applications in medicine, agriculture, environment and industry, particularly in the fields of drug discovery, diagnostics, biomanufacturing and metabolic engineering.
Its core relies on cell-free protein synthesis (CFPS), which uses the cellular machinery for transcription, translation and energy regeneration extracted from a cell, together with other components, to perform biomolecular reactions in a reaction vessel (Figure 1) [1].
The open nature of the reaction system enables to tune and optimize the synthesis conditions, permitting on-demand production of proteins with specific characteristics, in a variety of environments, including the interior of liposomes (to artificially create a cell) and microarrays (for protein screening) (Figure 1).
Furthermore, the system allows active monitoring and rapid sampling of the reaction medium, which offers enhanced control of the synthetic process. Since no time-consuming cloning steps are required, and the procedure can be automated, the whole process can be carried out in a shorter time, compared to cell-based systems [2,3].
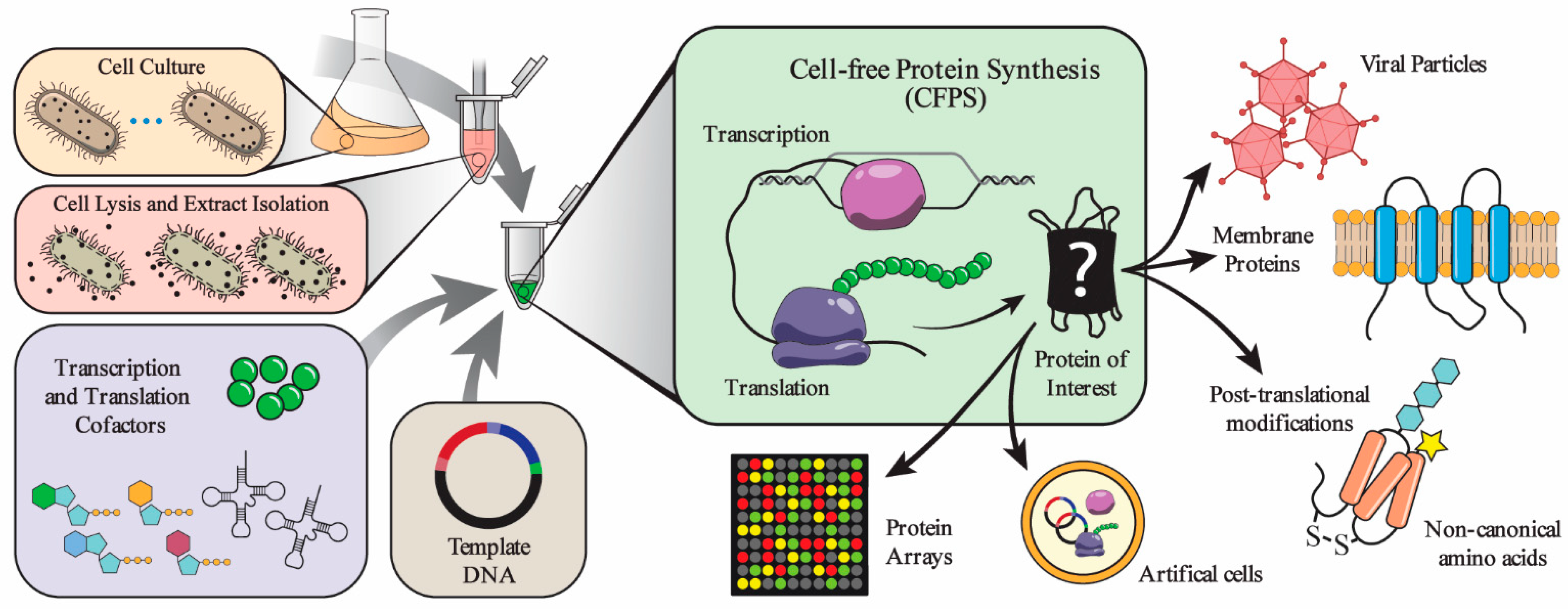
Figure 1 –Required components and examples of applications of cell-free protein synthesis systems [1]. Reproduced with permission.
CFPS systems allow to produce a great diversity of proteins, such as membrane proteins, collections of proteins to make viral particles and proteins with modifications like glycosylation, disulfide bonds and non-canonical amino acids (Figure 1).
Trends in CFPS from 1990 to 2020
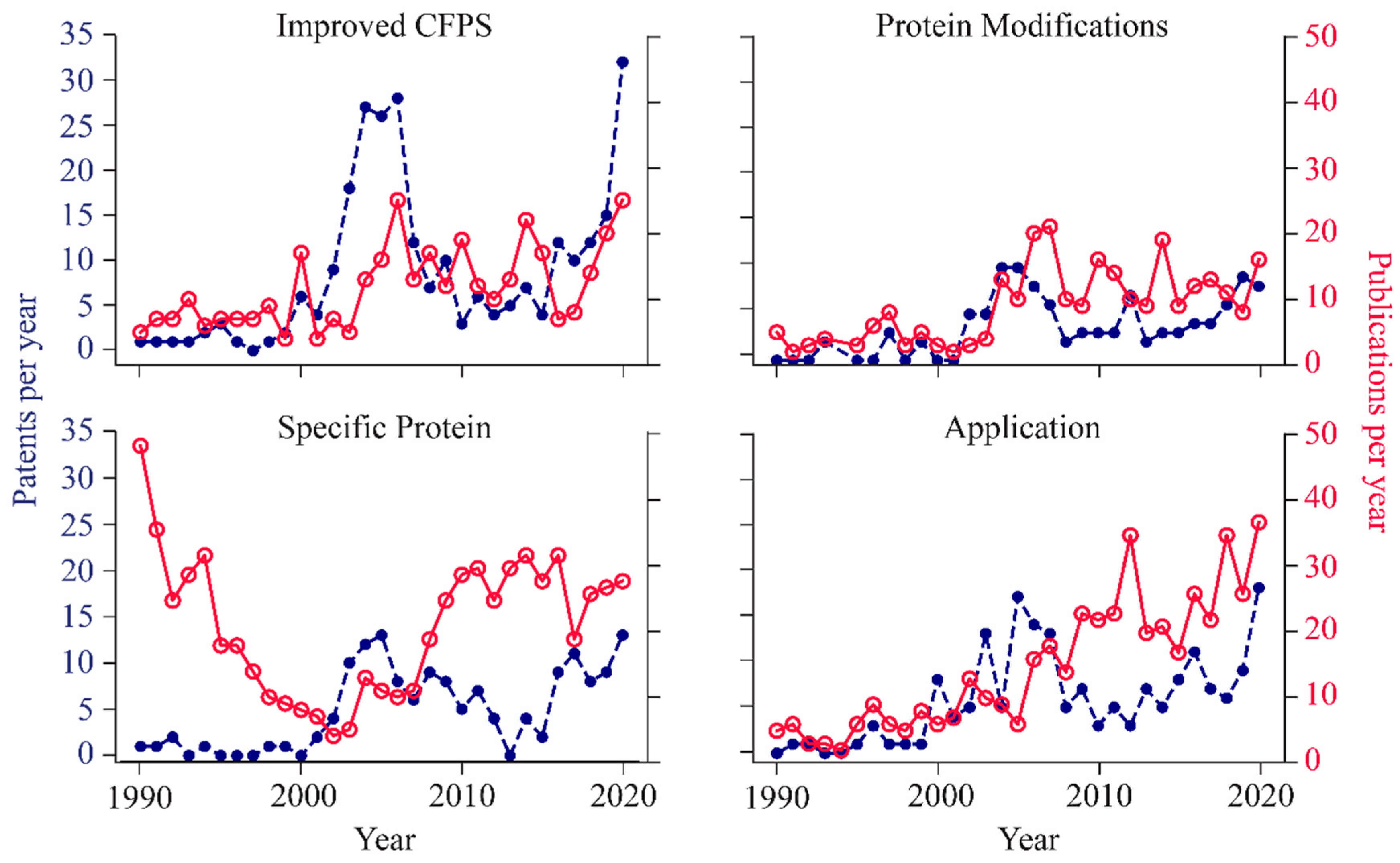
Trends in CFPS have been assessed in the period of 1990-2020, based on patent and publication counts worldwide [1], and can be grouped in the following categories:
(1) improvements in CFPS,
(2) protein modifications,
(3) production of difficult-to-express proteins, and
(4) development of novel applications using cell-free synthesized proteins.
For all trends, an increase in the number of patents and publications has been evidenced in the last decade (Figure 2). Improvements in CFPS include increased access to commercial kits, generation of extracts from non-model organisms and development of methods to optimize protein yields. The synthesis of proteins with modifications has also been a trend, by including mechanisms for post-translation modifications, such as disulfide bonds, glycosylation and the incorporation of non-canonical amino acids.
These modifications result in increased protein functionality allowing, for example, non-natural site-specific chemistries or fluorescent labelling. Production of specific proteins that are, otherwise, challenging to synthesize, has also been a trend in CFPS, for instance therapeutic and membrane proteins.
Finally, novel applications of proteins produced in cell-free systems have been also the focus of researchers, including the development of microfluidic systems for protein arrays, incorporation into artificial cells and the construction of kits for paper-based detection of pathogens or small molecules.
Figure 2 – Patents and publications per year, for the diverse trends in CFPS development [1]. Reproduced with permission.
While, in the 90s, CFPS was mainly dedicated to the synthesis of specific proteins, particularly viral particles, the focus has moved in recent years to the production of membrane-bound proteins, protein libraries, therapeutics and diagnostics.
The improvements in CFPS achieved in the decade 2000-2010 allowed to extend reaction durations, to reduce costs and to enhance protein functionality, which resulted in an increased availability of CFPS systems and rising curiosity to innovate in the other categories.
The applications with greater commercial interest include the production of therapeutic proteins and bioactive molecules such as conjugate vaccines and antibody analogs, point-of-use testing for pathogens and water contaminants, inexpensive platforms for biology education and the discovery and optimization of enzymes to make commodity chemicals, natural products and biomaterials. Although some difficulties persist, namely the price and scalability for industrial production volumes, the improvements made in small-scale CFPS systems enabled to reduce the design-build-test cycle, therefore allowing the rapid screening of protein libraries.
Conclusion
To conclude, CFPS is a matured area of cell-free synthetic biology, both in system development and applications, as shown by the increasing research and industrial implementation.
In the near future, a focus on quality control will be critical to provide cell-free products for practical uses, particularly on-demand therapeutic products and point-of-care detection. Reduction of costs and the ability to scale up reactions will enable the incorporation of these systems into routine lab workflows. The ability to readily store, distribute and activate freeze-dried cell-free systems by adding water will permit on-demand biomanufacturing and point-of-care diagnostics.
Finally, the creation of educational kits and courses will enable to increase the exposure of CFPS technology and allow to expand its perception.
Written by Luísa Silva, PhD, and scientific experte.
Synthelis
Since 2011, Synthelis has developed a proprietary cell-free expression system combined with a strong know-how to define optimized CFPS conditions to produce almost any protein of interest. If you have a project requiring rapid and efficient protein development, please contact us…
Sources
[1] Meyer C., Nakamura Y., Rasor B.J., Karim A.S., Jewett M.C., Tan C. 2021. Analysis of the innovation trend in cell-free synthetic biology. Life 11:551.
[2] Gregorio N.E., Levine M.Z., Oza J.P. 2019. A user’s guide to cell-free protein synthesis. Methods Protoc. 2:24.
[3] Silverman A.D., Karim A.S., Jewett M.C. 2020. Cell-free gene expression: an expanded repertoire of applications. Nat. Rev. Genet. 21:151-170.