Do you have a protein you want to express? Are you wondering which technology will provide you with the best results? Have you considered turning to a cell-free system? In comparison to traditional protein expression systems, cell-free protein synthesis can provide a competitive solution in terms of speed, efficiency and adaptability.
A recombinant protein is a protein produced by a cell whose genetic material has been modified by genetic recombination.
A gene encoding a protein of interest is introduced into the cell of the producing species.
Recombinant protein market is rapidly developing with ever increasing needs, but particularly for pharmaceutical companies seeking relevant targets and new biocandidates. To meet the growing demand, there are different protein expression systems. The cell-based Escherichia coli approach was widely regarded as the most popular recombinant protein expression system, although in recent years many other robust systems have emerged offering new choices and opportunities to the scientific community.
In times gone by, cell-free technology was referred to as an alternative protein expression method, which both pharma and academics alike turned to as a last port of call. Nowadays, this mentality has drastically changed because of improvements in the technology over recent years. Cell-free protein synthesis based on coupled transcription/translation systems with linear (PCR fragments) or circular DNA templates is now as efficient and successful as other, more well-known, expression systems and in many cases provides a better solution.
Contrary to the myth, cost is not a limiting factor with prices capable of competing with the other most commonly used methods at both small scale and industrial levels. Often the procedure is even less expensive thanks to increased versatility in the optimization steps resulting in higher protein yields, especially in the case of difficult or complex proteins.
How recombinant protein synthesis works in a cell-free system?
Cell-free protein synthesis consists of using cellular transcription and translation machinery outside of the cell. Cellular extract in a test tube provides the components needed to generate a protein from a DNA sequence. This lysate creates an artificial replica of the internal cellular environment containing the necessary elements for recombinant protein expression: RNA polymerase, ribosomes, tRNAs and enzymes.
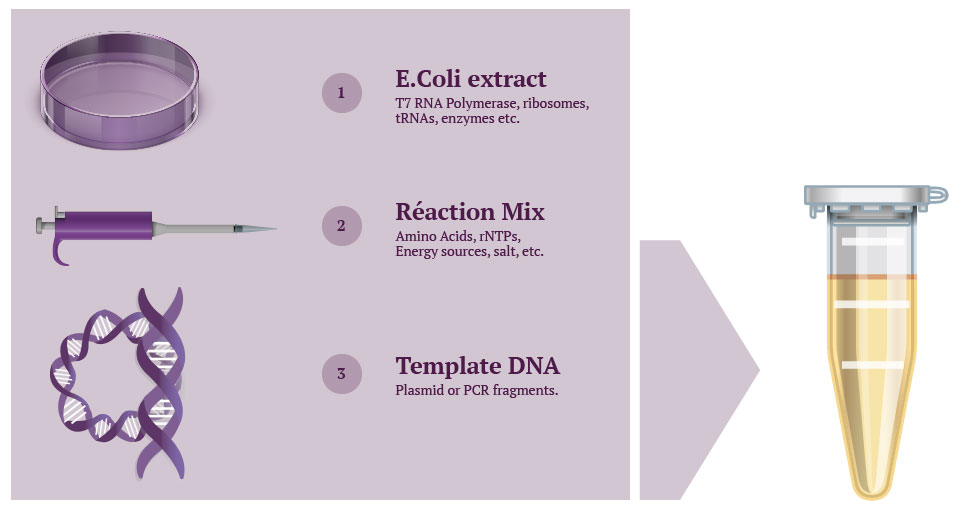
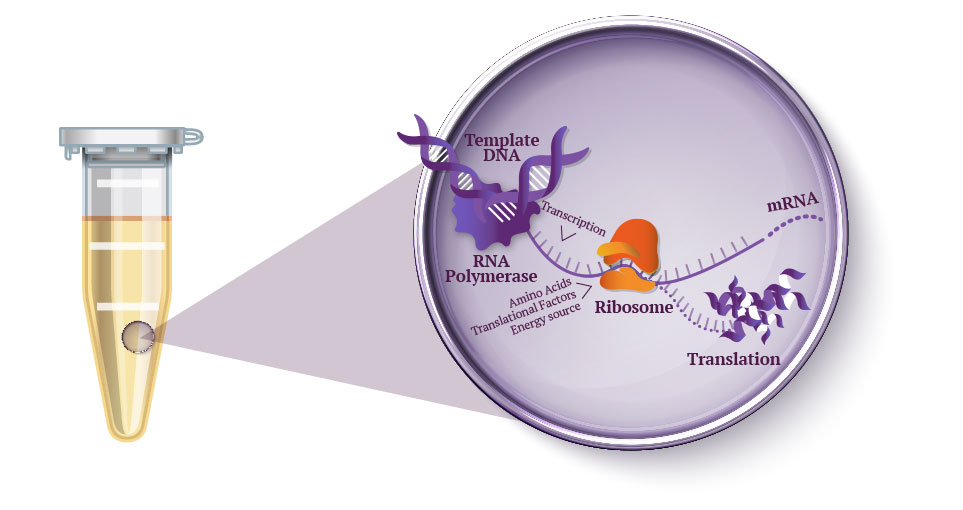
Cell-free protein synthesis uses cell extract and a reaction mix to express proteins from a DNA template (step 1) by performing cellular protein transcription and translation process inside a test tube (step 2).
A reaction mix mainly containing amino acids, nucleotides and sources of energy is added separately, meaning quantities can easily be modified and thus optimized – hence the versatility of the technique. Then, when the genetic template is added, the machinery gets to work transcribing it into mRNA, which in turn is translated into the desired cell-free expressed protein.
Cell extract can be stored for up to a year, streamlining the process. Within hours of thawing, all that is required is addition of the reaction mix and DNA sample.
Using a cell-free system in this way provides several advantages over traditional in vivo methods. Since it is an open system, it facilitates monitoring and manipulation of both the protein synthesis apparatus and the biochemical environment. The technology can be scaled-up too, proving effective at producing high yields of protein for pharmaceuticals.
What are the advantages of cell-free protein synthesis over an in vivo E. coli system?
Cell-free systems are rapid, yes. But that is far from the only advantage. For starters, expression of toxic proteins is not limited in an in vitro system like it would be using in vivo. Proteins that are toxic to the host cell will not be toxic in a lysate resulting in a much higher expression yield and thus quantity of produced protein.
For screening purposes, cell-free technology is ideal because of its capacity to express a multitude of genes in parallel and in a very short timeline. Indeed, the process allows us to express up to 800 genes in vitro per week; and even more if the process is automated. High throughput like this is much more difficult and intense to achieve with an in vivo system. On the other hand, cell-free expression is well adapted to small-scale testing of various conditions with minimal expenditure of reagents.
In structural biology, cell-free provides greater control over the expression parameters. It ensures the correct folding of the protein for analysis but also prevents the product from aggregating. Also, expression levels are not limited by the capacities of the host cells in cell-free systems so techniques like X-ray crystallography can require 5 mg or more of protein. At the end, cell-free systems guarantee you a quick, simple and value-for-money solution for this type of application.
Cell-free technology provides the ideal solution for fields such as synthetic biology too, where the living nature of in vivo system can hinder the protein produced. For example, in the case of introducing isotope-labelled or unnatural amino acids, the protein may be rejected or unfavored by an in vivo system.
Other instances include protein delivery such as proteoliposomes, production of which is not possible in a one-step reaction using a closed system. Cell-free renders such processes feasible and efficient, providing greater overall results.
Why choose a cell-free expert?
Whilst cell-free kits exist, they can result in disappointment. This reality has no doubt contributed to the belief that “cell-free doesn’t work very well”. As with any pre-fabricated kit, you hand over much of the control of essential parameters to the supplier, who has no prior knowledge of your specific case. At Synthelis, with 14 years of experience, we have developed:
– A strong expertise in cell-free technology for customized projects, because we know that every protein is different and that the conditions required to obtain good results need to be addressed on a case-by-case basis.
– A patent which allows us to produce the most challenging proteins (GPCR, ion channel, cytotoxic) with a high level of quality.
– A quality management system which ensures reproducible, robust and optimized cell extract to obtain proteins:
– With a proper folding.
– With a high expression level.
– Without formation of inclusion bodies or protein truncated forms.
Furthermore, we provide a hands-on service with our experts who can adjust the parameters or additives used to best suit your needs, therefore ensuring success in achieving greater expression rates and higher protein quality. Recently, we successfully produced 10 mg of a membrane protein in as little as just two days!
At the end, we work in close collaboration with a network of reliable partners such as the synchrotron platform (ESRF), as well as the neutron source thanks to ILL and the high throughput crystallization platform (HTX) at EMBL, in Grenoble. We can take you through the required quality control steps and can go even further in the analysis of your product if you require. A truly hands-on service that takes you all the way.
Sources
Rosenblum, Gabriel and Cooperman, Barry S. (2014), Engine out of the chassis: Cell-free protein synthesis and its uses, FEBS Letters, 588, doi: 10.1016/j.febslet.2013.10.016.
Carlson ED, Gan R, Hodgman CE, Jewett MC. Cell-free protein synthesis: applications come of age. Biotechnol Adv. 2012 Sep-Oct;30(5):1185-94. doi: 10.1016/j.biotechadv.2011.09.016.
Niederholtmeyer H, Sun ZZ, Hori Y, Yeung E, Verpoorte A, Murray RM, Maerkl SJ. Rapid cell-free forward engineering of novel genetic ring oscillators. Elife. 2015 Oct 5;4:e09771. doi: 10.7554/eLife.09771.