Cell-free production of adeno-associated virus protein particles – Expanded horizons for gene therapy and vaccine development
Virus-like proteins (VLPs) are non-infectious polymeric nanoparticles formed from virus-coat proteins but deprived of genetic material. These features have enabled their successful use in several applications such as vaccines, gene therapy, and drug delivery systems [1]. Among the diverse VLPs developed so far, recombinant adeno-associated viruses (rAAVs) are increasingly used in gene therapy and vaccine development due to their low immunogenicity and lack of pathogenicity [2]. AAVs are nonenveloped single-stranded DNA viruses, and their genome encodes for three structural capsid proteins VP1, VP,2, and VP3. VP3 can self-assemble into capsids without the other capsid proteins.
Limitations of Traditional rAAV Production
Traditionally, rAAVs have been produced in cell-based systems using mammalian, insect or bacterial cell lines, but these methods are time-consuming and have high costs, generating low titres of proteins in impure forms [3].
Cell-Free Production as an Alternative Approach
Cell-free production emerges as a valuable alternative for rAAV synthesis, providing rapid production with a simple workflow. Besides, due to its open nature, it can be easily manipulated to adapt to the protein of interest, by introducing enzymes and post-translational modifications that may not be feasible in prokaryotic cell-based systems.
Recent Advances in Cell-Free rAAV Synthesis
A recently proposed cell-free method for the synthesis of rAAVs clearly demonstrates the advantages of cell-free protein synthesis (CFPS) for this type of protein. The method consisted on an E. coli-based cell-free system for the production of rAAV serotype 5 VP3 VLPs (rAAV5 VP3 VLPs) (Figure 1) [4].
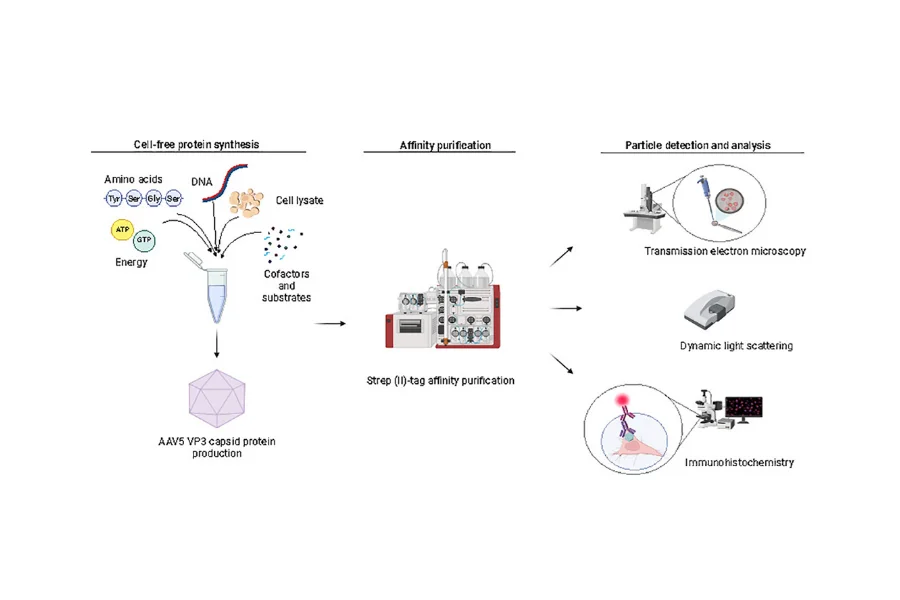
Figure 1 – Cell-free production, purification and characterization of recombinant AAV5 VP3 VLPs. Reproduced from [4].
The authors used an E. coli cell-free lysate generated using BL21 Star, and T7 RNA polymerase was supplemented in addition to the endogenous T7 RNA polymerase in the cell lysate to increase protein yields. Constructs with or without Strep-tag II were expressed (Figure 2).
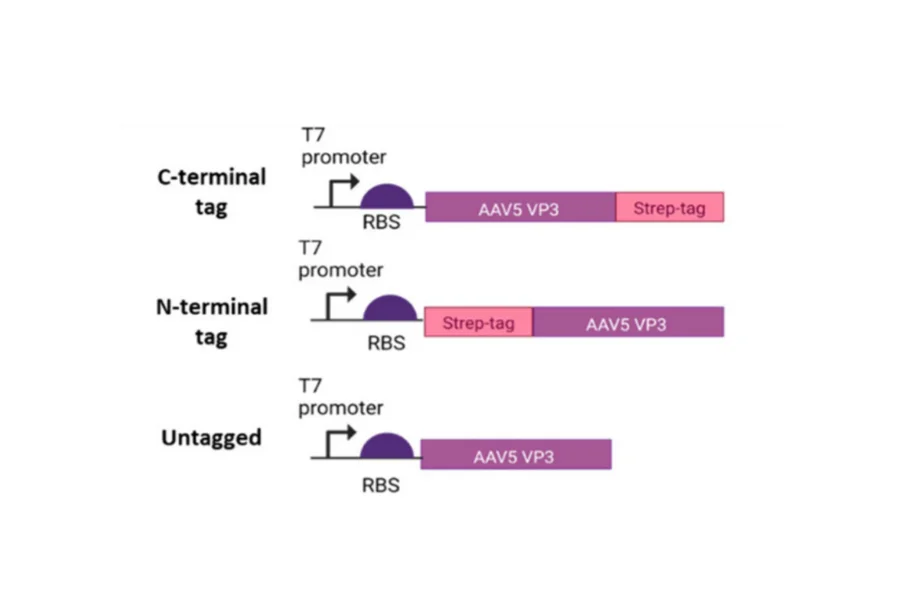
Figure 2 – AAV5 VP3 gene designs that were expressed in the cell-free system. Reproduced from [4].
Optimization of rAAV5 VP3 Production
Protein produced after 16 h CFPS was successfully identified by Western blot, using the primary antibody B1 which recognizes unassembled monomeric AAV capsid protein. The impact of temperature on the production of soluble protein was assessed by testing temperatures from 18 °C to 37 °C, and it was found that lower temperatures favored the synthesis of the soluble protein, with the untagged variant containing around 90% more soluble protein at 18 °C than at 37 °C. This could be due to increased protein misfolding at higher temperatures.
Purification and Characterization of rAAV5 VP3 VLPs
Affinity chromatography of N-terminally Strep(II)-tagged VP3 allowed the efficient isolation of the variant with minimal processing and enabled further characterization of the protein particles. The fact that the protein was largely produced in soluble form facilitated its purification. The estimated production yield was 2.98e9 capsids/mL of cell-free reagent, and the obtained particles had ~20 nm diameter, as expected for an AAV5 particle. The biological activity of the produced AAV5 VP3 VLPs was evaluated by testing their internalization into HeLa cells, and it was shown that the cell-free produced particles were efficiently internalized, meaning that they had the appropriate morphology for receptor-mediated endocytosis.
Potential Improvements for Enhanced Production
According to the authors, the relatively low purified capsid titers obtained can be increased by using refined purification techniques and by making adjustments in the cell-free system, namely by incorporating cofactors and additives to improve yield and capsid assembly. The cell-free method also offers the possibility for cargo loading or in vitro gene packaging after capsid assembly. Furthermore, the developed cell-free system constitutes a valuable high-throughput screening tool for alternative rAAV serotypes and mutants, facilitating the rapid prototyping of self-assembling particles.
Broader Applications of CFPS for VLP Production
In a more general sense, the advantageous application of cell-free synthesis for the production of VLPs has been widely proved in diverse applications such as the production of hepatitis B core VLPs [5,6] and human noroviruses VLPs [7] to be used as vaccine candidates.
The developed cell-free systems for VLP production showed improved performance over cell-based methods, facilitating the rapid screening of protein variants.
Additionally, CFPS enables better control of the reaction conditions, namely the pH and the redox state for the formation of disulfide bonds, which is a critical aspect in VLP synthesis, affecting the stability of the synthesized particles [8]. Furthermore, in cell-free systems, VLPs are purified in a single step, significantly reducing the cost of production [9] and the production time [5,7].
CFPS has allowed several improvements in VLP synthesis, such as surface functionalization of VLPs by direct conjugation using azide-alkyne click chemistry [10], expansion in VLP bioactivity [11], reduction of VLP intrinsic immunogenicity and increase in VLP solubility and assembly [12].
Written by Luísa Silva, PhD and, scientific expert.
Synthelis
With over more than 15 years of experience in cell-free technology, the Synthelis team can assist you if you wish to test VLP production using a cell-free system. Feel free to contact us if you have such a project.



