The future
of production
is bio.
Cell-Free fields of application
Biomanufacturing consists primarily of synthesising biological products by multiplying cells that incorporate the elements necessary to produce proteins and other specific molecules, thus enabling the mass production of biomolecules of interest.
Applications
The production of biopharmaceuticals is currently a major growth area for biomanufacturing and includes the synthesis of antibiotics, monoclonal antibodies, vaccines, enzymes, modified human proteins, growth factors and viral vectors, for the treatment of various diseases, for which these products have high specificity and efficacy, associated with lesser side effects.
In addition, bioactive natural products (BNP) may be also bioproduced. BNP are compounds that are naturally synthesized by animals, plants or microorganisms, and may act as building blocks, coenzymes, host-defense factors, mediators of gene-expression, cellular signaling or homeostasis. They have been used as pharmaceuticals, nutraceuticals, agrochemicals and cosmetics.
Among them, we can refer antineoplastics (paclitaxel), anti-tumour agents (mithramycin), immunosuppressants (rapamycin) and antiparasitics (ivermectins). Considering the high global demand for these molecules, their obtention from the corresponding natural sources is not viable due to the limited availability, the complex and expensive extraction and isolation methods, and the low yields. On the other hand, their chemical synthesis in the laboratory may also be problematic due to the high cost of production and complex stereochemistry. By using in vitro cultures of cells to synthesize the biomolecules, production yields are increased and production costs are decreased [1].
Technology
The therapeutic molecules are manufactured from living organisms such as animals, plants, microorganisms and/or by biotechnological methods, including recombinant DNA techniques and cell extracts. Non-human mammalian cells are commonly used on an industrial scale, although some human cell lines can be used in vitro.
In addition, cell-free systems based on the use of cell lysates are becoming an important alternative to cell-based systems for the synthesis of various therapeutic biomolecules, as they offer several distinct advantages, such as the possibility of producing proteins that would be difficult or impossible to obtain in living systems, due to their cytotoxicity or due to particular structural characteristics.
Trends and challenges
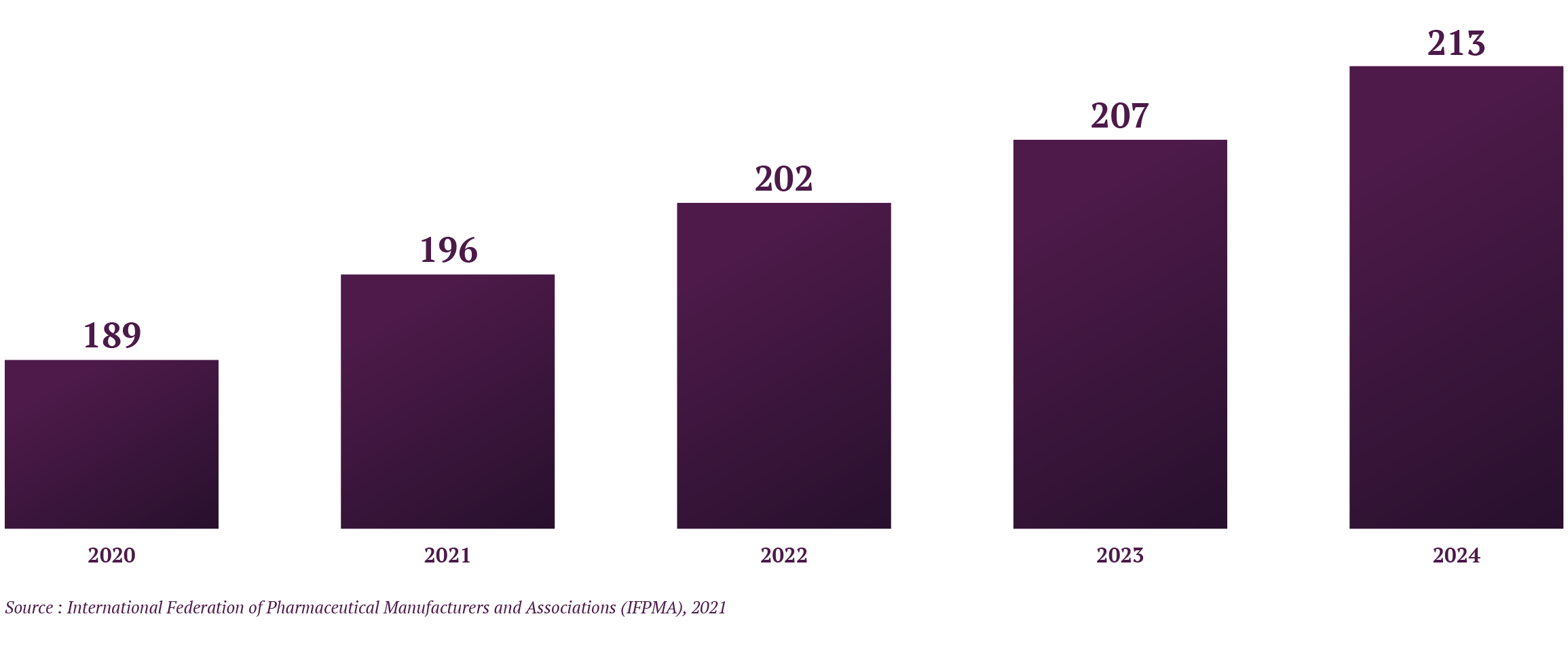
Over the last 30 years, enormous investment and progress has been made in biomanufacturing technologies (Graph 1) and various biopharmaceuticals have been launched on the market. The bioproduction of monoclonal antibodies, for example, has already reached a point of maturity. Furthermore, average yields achieved by commercial-scale manufacturing processes with mammalian systems lie in the range of 70-80%. Downstream processing technologies (including purification) have become more efficient, enabling smaller overall volumes and single-use approaches. It is now easier and faster to establish stable cell lines and cell banks. Nevertheless, the biomanufacturing of certain cell and gene therapies still needs to be improved to reach full maturity.
For the near future, the trends in bioproduction include:
. developments in the technology, such as single-use bioprocessing systems and continuous bioprocessing operation modes;
. development of new gene and cell therapies;
. and the implementation or increased use of artificial intelligence and big data analytics.
The innovations in technology aim to increase automation, as this will maximize bioproduction and minimize capacity constraints. Single-use bioprocessing systems enable to reduce the risk of cross-contamination, increase the flexibility of scaling production and allow a faster setup, enhancing the efficiency and cost-effectiveness of bioproduction. Continuous bioprocessing permits the uninterrupted production, reduces batch-to-batch variability, improves product consistency and lowers production costs [2]. Innovative production methods are needed in order to meet the demands for the production of gene and cell therapies, namely strict quality control and specialized manufacturing techniques [3]. Artificial intelligence can optimize bioprocesses, predict potential failures and analyze vast datasets, leading to more efficient and consistent production. Machine learning models can assist in drug discovery and protein engineering, and data-driven methodologies allow to save time and resources in bioproduction [4].
Graph 1 : Estimated Spending in Biopharmaceutical Research and Development (in USD billion), Gloabl, 2020-2024
Cell-free systems and bioproduction
Although bioproduction has been based on the use of cells to synthesize relevant biomolecules, cell-free systems may also be employed in biomanufacturing, and can even exceed the performance of cell-based processes. Cell-free protein synthesis (CFPS) is particularly well-suited to synthesize “difficult-to-express” proteins such as cytotoxic, misfolded or nonsoluble proteins, which are common among biopharmaceuticals and immunobiological products.
The open nature of the system allows the addition or removal of substrates and products and the online monitoring of the reaction, for greater control of the process. Furthermore, cell-free technology bypasses cell culturing steps of in vivo methods, accelerating the production and reducing costs. Considering industrial bioproduction, CFPS has the ability to scale up and scale down and enables high throughput production [5,6]. Furthermore, it allows for on-demand production, according to consumers’ needs [7], showing enhanced flexibility versus cell-based biomanufacturing.
Portability of cell-free systems and the possibility to use lyophilized cell extracts allow for point-of-care treatments [8,9]. Moreover, the CFPS attractive features open the possibility to shift from the traditional hydrocarbon-dependent processes towards more sustainable biomanufacturing of chemical and biological molecules. It also represents an advantageous alternative in situations where traditional supply chains are compromised by global pandemics, natural disasters or conflict [10]. CFPS has already been used for the bioproduction of biopharmaceuticals, immunobiological and bioactive natural products.
Written by Luísa Silva, PhD and scientific writer.
Publications
References
We look
forward to
your questions
Even
a protein
needs
to express
its potential.
Cell-Free Systems Applications
